42
Vol. 66, No. 2 2015
Northeast Florida Medicine
CME
The median time to failure was six months from di-
agnosis. Of the eight total recurrences (including two
local recurrences in a single patient), six were salvaged
and currently have no evidence of disease. Two patients
are alive with active disease.
Survival
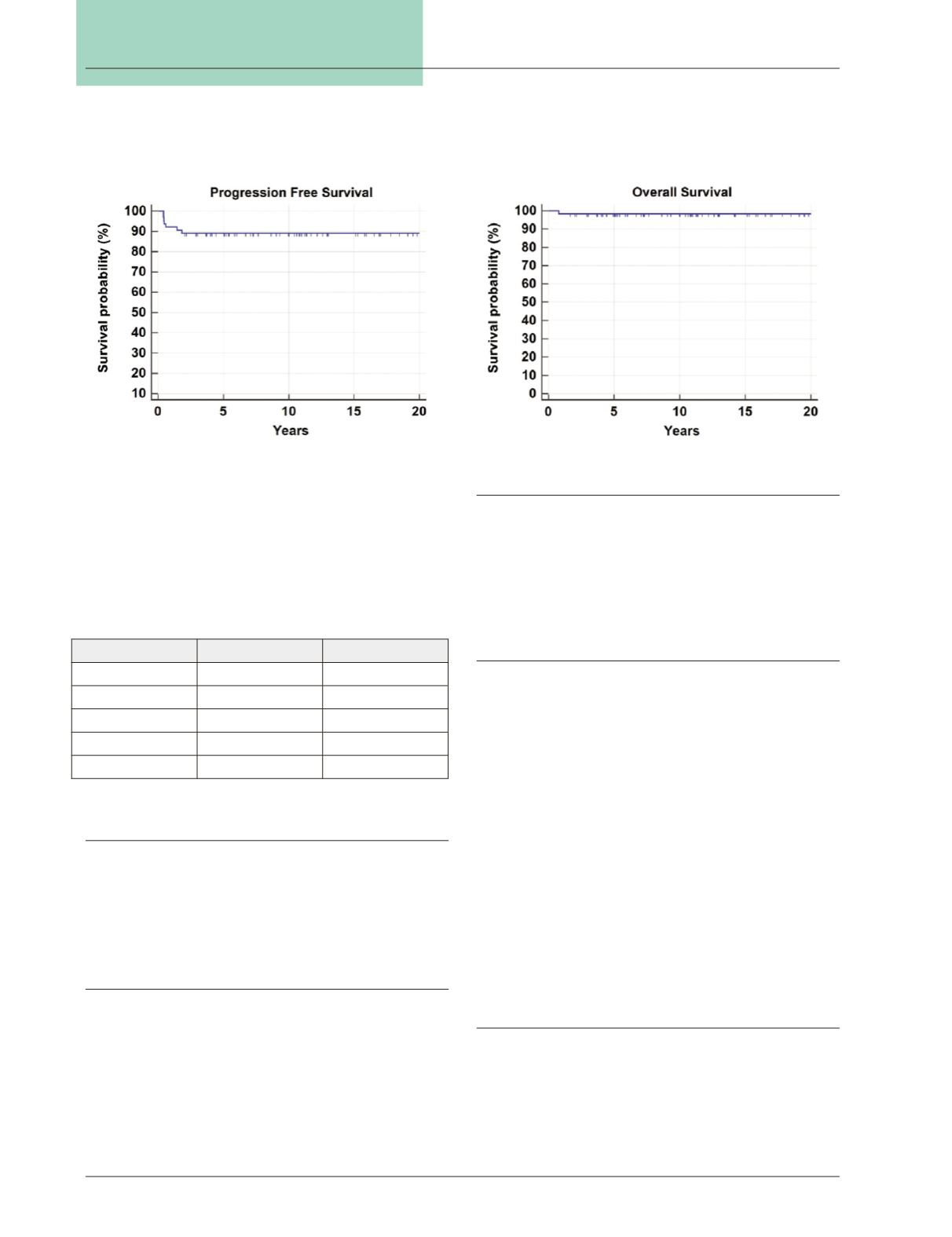
Progression-free survival was 89 percent (Fig. 1). 63 of
the 64 patients are still alive, giving an overall survival of
98.4 percent (Fig. 2). The single patient who died had
stage IV disease at diagnosis and experienced a recurrence
prior to death.
Conclusion
Children withWilms tumor can have excellent long-term
outcomes when managed per NWTS/COG protocols.
After 11 years of median follow up, PFS and OS were both
excellent at 89 percent and 98.4 percent, respectively.
Discussion
Based on recent COG protocols, patients are not treated
based on stage alone, but are placed into risk categories
that determine management. The high risk group includes
patients with unfavorable histologies, including focal
and diffuse anaplasia. All other risk groups include only
favorable histology (FH) Wilms tumors.
Very Low Risk
Patients are classified as very low risk if they have stage I
disease, are less than 2 years old, and have small tumors. On
the most recent COG protocol (AREN0532, now closed to
accrual), these patients were managed by surgery, followed
by observation. Patients were required to undergo surgical
lymph node evaluation to be enrolled in this study. This
arm of the study was based on results fromNWTS-5 which
attempted to decrease treatment related toxicity for very
low risk patients. Initial analysis of NWTS-5 suggested a
lower-than-expected EFS (86 percent at two years) and the
very low risk arm was closed early. With longer follow up
of NWTS-5, salvage rates were excellent in this population
and AREN 0532 observation was once again attempted.
As AREN 0532 closed to accrual in October 2013, current
very low risk patients are generally offered standard EE4A
chemotherapy (actinomycin-Dand vincristine) until results
of AREN 0532 become available.
Low Risk
Patients are classified as low risk if they have no LOH
1p/16q and have stage I or II disease. The standard man-
agement for these patients is surgery, followed by EE4A
chemotherapy. No therapy-based trials are currently open
for this cohort of patients; however, they are enrolled in
AREN 03B2, a tumor classification, biology, and banking
Stage
PFS (%)
OS (%)
I
100
100
II
80
100
III
93
100
IV
91
91
V
100
100
Table 3: Progression-free survival and overall survival
rates by stage for patients treated at a single institution.
Figure 1:
PFS for all patients treated at single institution.
Figure 2:
OS for all patients treated at single institution.


