
Winter 2015
NCRWA.COM33
feature
addition to tracking the calibration itself,
the LIMS can store reference material
certificates that demonstrate the quality of
the materials used.
Staff Performance
In addition to instrument error, lab results can
also be invalidated by human error. Human
errors in the lab originate from issues in one
of three broad categories: training, process
and data management. A defensible lab result
must be able to demonstrate that the lab staff
that produced it did not inadvertently make
errors in any of these areas.
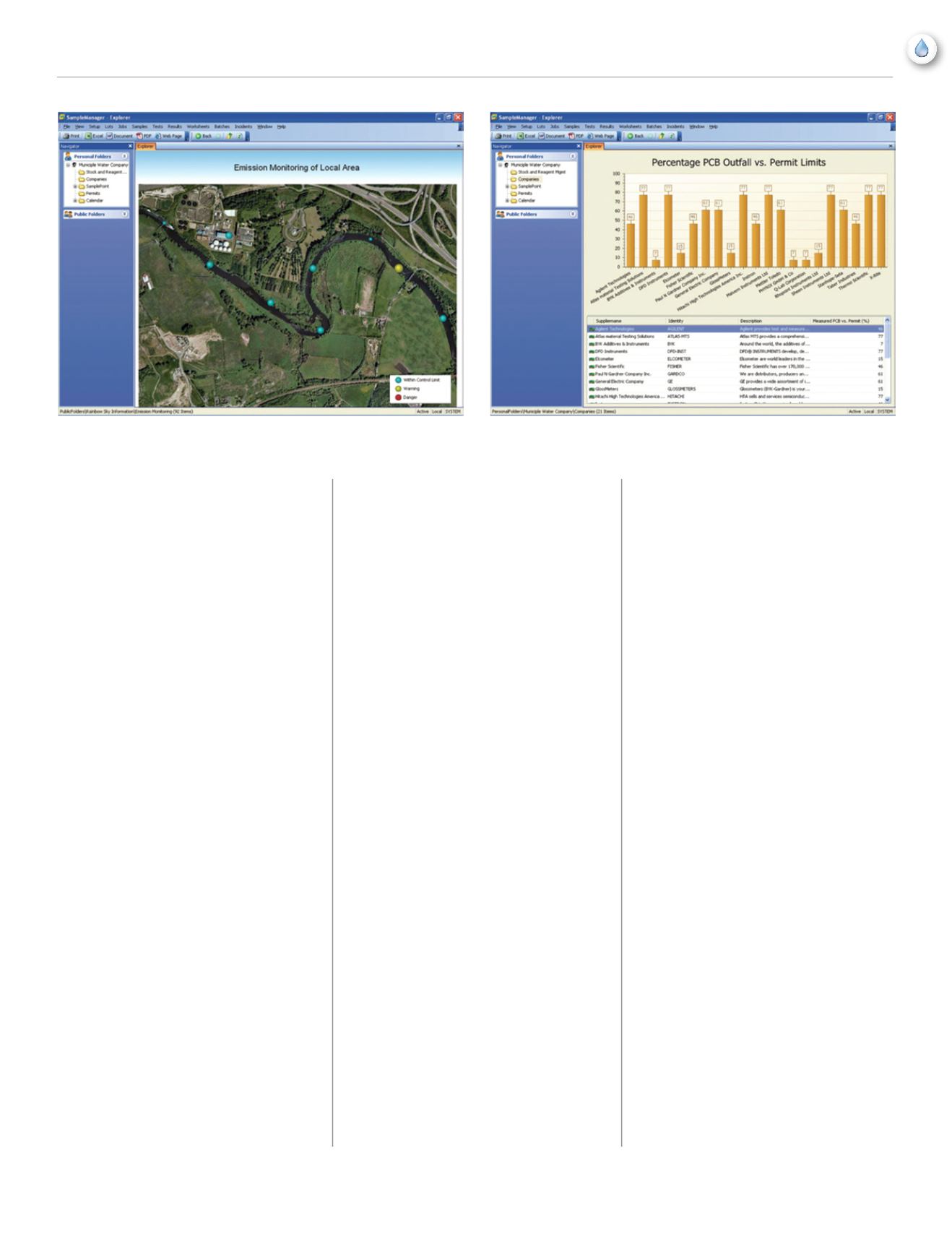
Data Visualization allows users outside the
laboratory to monitor field collection sites
quickly and easily within the LIMS.
Staff Training:
Training for each employee must
be closely monitored to ensure that they’re
up-to-date; otherwise results from any
part of an analytical process in which they
participate could be questioned. Maintaining
accurate and up-to-date staff competency
records is an onerous process that can be
simplified by storing all personnel training
records, certifications and permissions
within the LIMS.
Process Quality Assurance: Human error is
one of the most common causes of failure
in any process: small errors in the execution
of a GC analysis can render a result invalid.
To prevent this, labs must be able to
comprehensively monitor the execution of
laboratory processes and reference them to
individual results.
A LIMS achieves this by storing and
automating SOPs to walk staff through each
analytical or operational process. As staff
members progress through the steps of an
SOP, they mark their progress in the system,
creating real-time records to document that
they executed everything correctly. Process
errors can also be reported to a lab manager in
real time, allowing them to correct problems
before erroneous results are even reported.
Dashboards provide graphical representations
of information to allow laboratory management
and analysts to visualize critical information in
various forms.
Data Entry and Transcription: Manual data
entry errors made by staff can significantly
affect lab results. Even if a process is nearly
entirely automated, a single manual data
entry error can make the result indefensible.
ALIMS helps alleviate data entry concerns by
automatically collecting and aggregating lab
instrument data where they can be accessed
by staff or used by other instruments in the
lab. A vendor-agnostic LIMS is particularly
important here because it’s capable of
interfacing with a broad range of instruments
from multiple vendors.
Conclusion: Complete Traceability
The areas covered above by no means
comprise the exhaustive list of variables
involved in a typical GC analysis, and this
is just one of many analytical tests a water
industry labmight undertake. But the example
illustrates the point that there are many ways
for a single experiment to go astray and lead
to a disputed result. But defending results is
about more than simply verifying inputs and
outputs; it’s about documenting complicated
processes rigorously at every step without
pulling staff away from important work.
A LIMS can provide complete visibility into
processes and the staff that execute them.
Moreover, it can direct processes in a manner
consistent with SOP and, of course, quality.
This complete traceability streamlines
laboratory operations and provides the
defensibility that’s so important to lab staff.
For water industry labs, defending results is
a way of life but the task is made much easier
with software that looks over your shoulder,
defending you every step of the way.
About the Author:
Trish Meek is Director of
Strategy, Informatics, for Thermo Fisher Scientific.
Dashboards provide graphical representations of information to allow laboratory
management and analysts to visualize critical information in various forms.
Data Visualization allows users outside the laboratory to monitor field
collection sites quickly and easily within the LIMS.
















